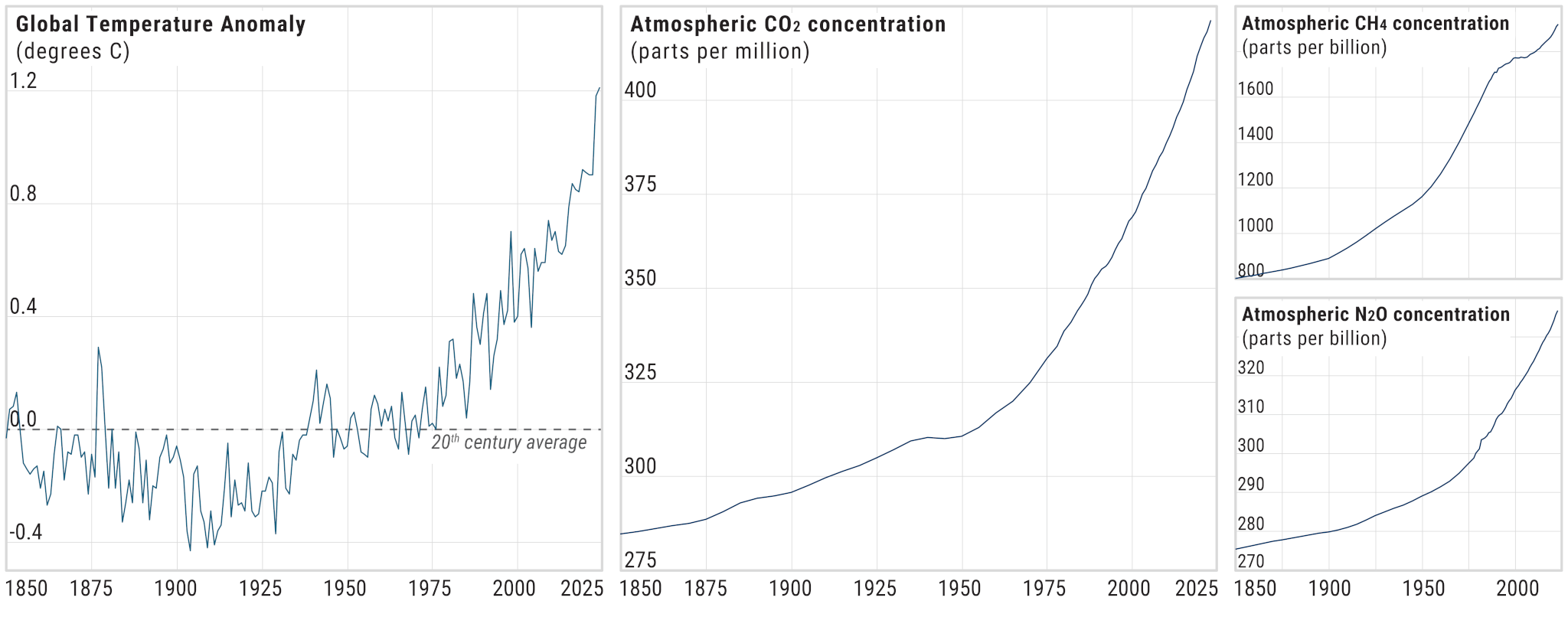How Impactful are Different Greenhouse Gases?
Carbon dioxide (CO2) is the most abundant greenhouse gas. Each year, hundreds to thousands of times more CO2 is emitted into the atmosphere by human activity than any other greenhouse gas. However, other gases like methane, nitrous oxide, and halogenated compounds (industrial-derived gases that contain fluorine, chlorine, or bromine) have an outsize greenhouse effect relative to their concentration because of two critical characteristics: their radiative efficiency and their atmospheric residence time.
Radiative efficiency is a greenhouse gas’s ability to absorb energy and radiate it back to Earth. Atmospheric lifetime is a measure of how long a gas stays in the atmosphere before natural processes remove it (e.g., through breakdown via chemical reactions, or cycling into the ocean or biosphere).
In order to compare the relative impact of different greenhouse gases, these characteristics are incorporated into a measurement called the Global Warming Potential (GWP). GWP is a measure of the radiative efficiency of each unit of gas (by mass) over a specified period of time, expressed relative to the radiative efficiency of carbon dioxide (CO2). GWP is often calculated over 100 years, though it can be done for any time period. Gases with GWPs greater than one will warm the Earth more than an equal amount of CO2 over the same period, and the higher the GWP, the greater the warming influence. A gas with a long lifetime, but relatively low radiative efficiency, might exert more warming influence than a gas that has a comparatively high radiative efficiency but leaves the atmosphere faster than the time window of interest. This is reflected in a higher GWP in the long-lived, low radiative efficiency gas.
The table below presents atmospheric lifetime and GWP values for the three most significant greenhouse gases and select examples of halogenated compounds (of which there are more than one hundred) from the Sixth IPCC Assessment Report (AR6) released in 2021. These values are periodically updated by the scientific community as new research refines estimates of radiative properties and atmospheric removal mechanisms (sinks) for each gas.